LIFETIME: Prospective primary data collection framework
The ConcePTION project has developed a standardized framework for long-term follow up of neurodevelopmental outcomes of children exposed to medicines during pregnancy. The LIFETIME framework tracks neurodevelopmental outcomes through to school age, providing an evidence-base for medicine safety in pregnancy.
About LIFETIME
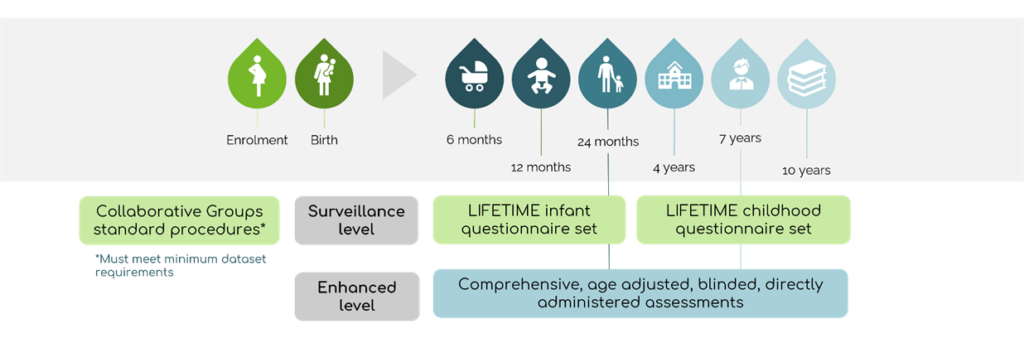
The LIFETIME framework collects prospective primary data in a standardized manner, and is designed to extend existing surveillance initiatives established for major congenital anomalies (for example, disease specific pregnancy registers and teratology information services). It has two levels of investigation: surveillance which is suitable for assessing large populations and, enhanced which is optimized for rarer used medications, new medications or where more detailed investigation is required.
LIFETIME offers four key benefits:
- It creates infrastructure and capacity through which pregnancy and breastmilk exposures can both be investigated.
- It provides a system for generating routinely collected neurodevelopmental data which is currently absent.
- It ensures real time data collection for new and rarely used medications allowing for important early data.
- It brings consistency and standardisation to data collection, allowing for larger international cohorts and more meaningful analysis.
The progress so far
LIFETIME has been developed by a team of experts in child development, clinical genetics, teratology, pharmacovigilance, statistics, and epidemiology. It is an ongoing project, delivered through a collaborative network of expert centres across Europe. So far, the LIFETIME framework has been utilized in four European centres and 1,200+ mother-child pairs have been recruited.
Want to know more about LIFETIME? Contact Rebecca Bromley at rebecca.bromley@manchester.ac.uk.
