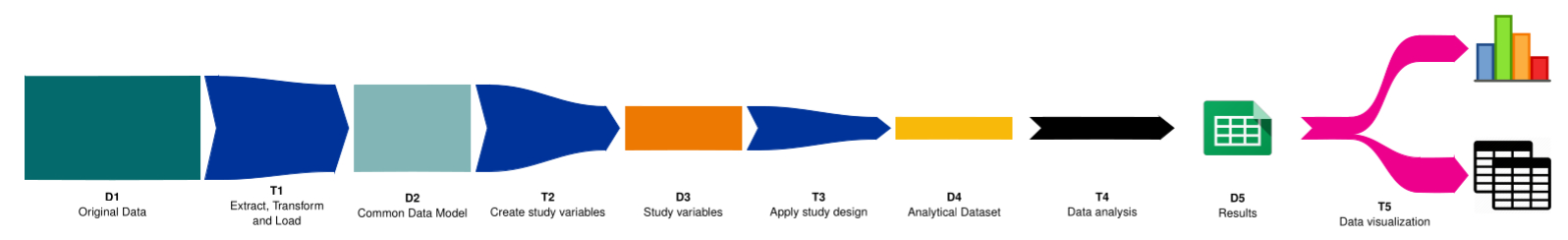
The IMI ConcePTION project has created a data pipeline for pharmacoepidemiology studies based on real-world data sources. The pipeline can be used to study the use and safety of medicines in pregnancy and lactation, but it is also applicable to a vast range of research questions. The pipeline represents the pathway of data transformations from original data to the figures and tables displaying the results of a study as a sequence of five steps.
A description of the ConcePTION data pipeline is available on a GitHub wiki, along with our Common Data Model documentation; catalogue of data sources; tools to support, extract, transform and load data sources to the ConcePTION Common Data Model; the INSIGHT real world data quality checks; the ConcePTION Pregnancy Algorithm; open-source functions to build and document study scripts; repositories of studies using the ConcePTION pipeline, and published studies based on the ConcePTION pipeline.
Do you want to learn more? Visit the ConcePTION pipeline wiki.