Meds4Mums2B: Mobile app
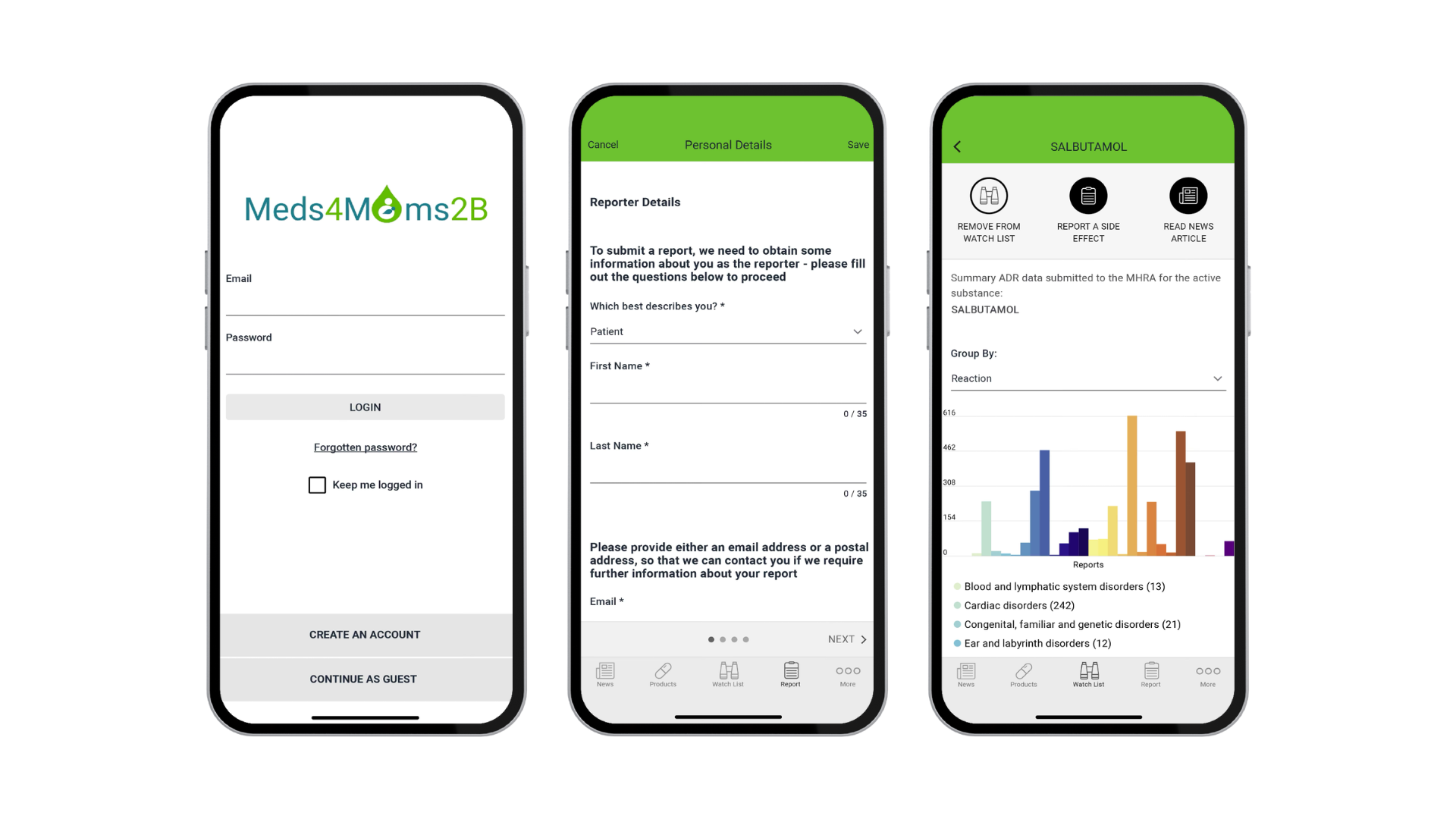
One objective of the ConcePTION project is to increase the volume, quality, and comprehensiveness of reports on exposure to different medicines in pregnancy and breastfeeding. One of the outcomes of our effort is the ‘Meds4Mums2B’ mobile application that is designed to provide verified, trusted information to parents and their health care professionals, and collect information about medicine use in the United Kingdom.
The app is developed in collaboration with the UK Medicines and Healthcare Products Regulatory Agency (MHRA), which will handle the case reports. Users in the United Kingdom will be able to both report the medicines they have used during pregnancy and breastfeeding and receive information on medicine safety through the app. By using the app, women and healthcare providers in the UK can help future mothers, by providing information about how their children develop. The MHRA will analyse the data that is collected through the app, and continue their efforts to describe the safety profiles of different medicines.
The app is connected to the MHRA news feed and filters information that is relevant for pregnancy and breastfeeding. The news feed will also be integrated with the ConcePTION MUMS knowledge bank. The app is a complement to existing systems for monitoring medicine safety, designed specifically for pregnancy and breastfeeding, and it is important to note that adverse events will reach the MHRA, even if they are not reported through the app. The data collection is secure. It complies with the GDPR and follows the MHRA and ConcePTION data management and privacy policies.
What is available for women outside the United Kingdom
The app can only handle information from the UK. The reason for this is the fact that different countries have different systems for collecting data for pharmacovigilance in pregnancy and breastfeeding. If you are not living in Great Britain or Northern Ireland, and have used medicines during pregnancy and breastfeeding, you can report to the company that makes the medicine (the Marketing Authorization Holder), or your doctor. In some European countries, there are also specialised, country-specific centres called Teratology Information Services. There is also the equivalent of the UK MHRA in every country that can receive your report. Want to know more about how to report? Go to our dedicated web page on how to report medicine use in pregnancy and breastfeeding.
Want more information?
The app was developed in the ConcePTION work package 2, focusing on improving the collection, analysis and interpretation of pregnancy pharmacovigilance data. The app is described in a paper published Open Access in Reproductive, Female and Child Health:
Alexe, A., Lewis, D. & Harrison, K., An innovative mobile app for the provision of medical information and collection of safety data on exposure to medicines during pregnancy or breastfeeding: An IHI ConcePTION contribution, Reproductive, Female and Child Health., (2024) Vol 3, Issue 1, https://doi.org/10.1002/rfc2.74
Download the app (for UK only)
The app is available to download on both App Store (iOS) and Google Play (Android) for mobile and tablet devices. Scan the QR code below to download the app.


